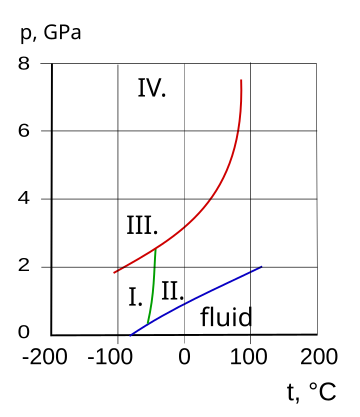
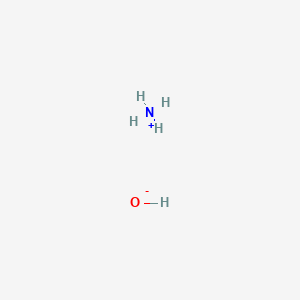
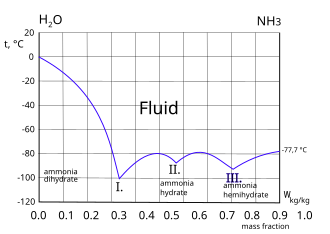
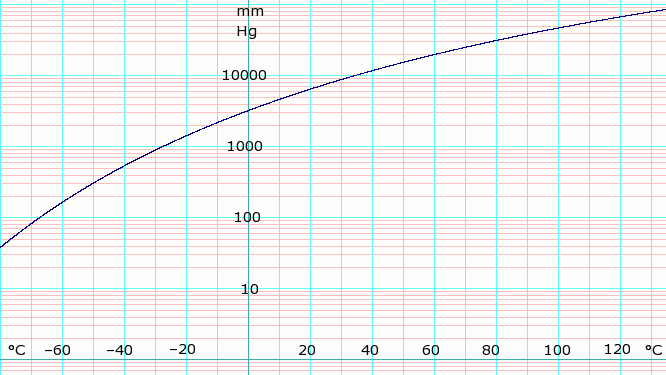
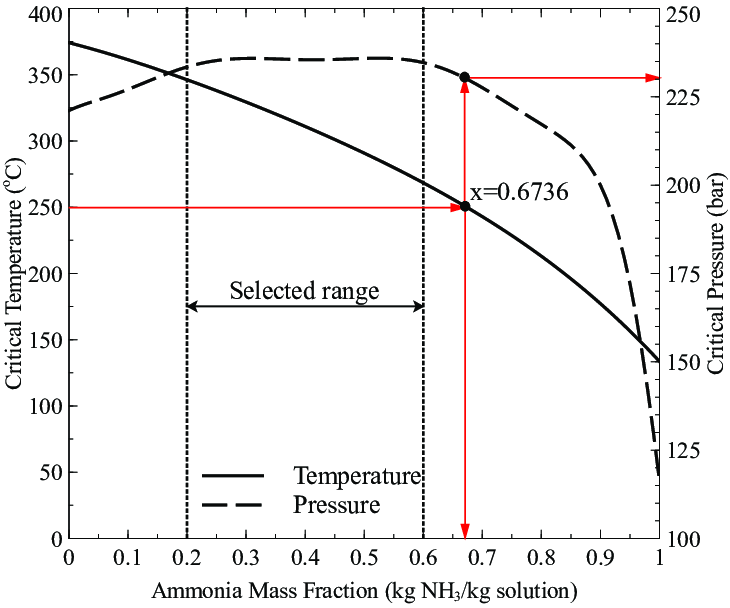
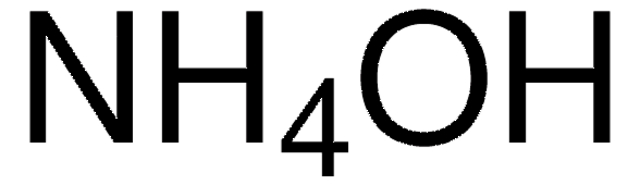Pressure-temperature diagram of ammonia in dependence on the filling... | Download Scientific Diagram

Vapour pressure measurements of ammonia/ionic liquids mixtures as suitable alternative working fluids for absorption refrigeration technology - ScienceDirect