thermodynamics - Does adding salt to water decrease the latent heat of vaporization? - Physics Stack Exchange
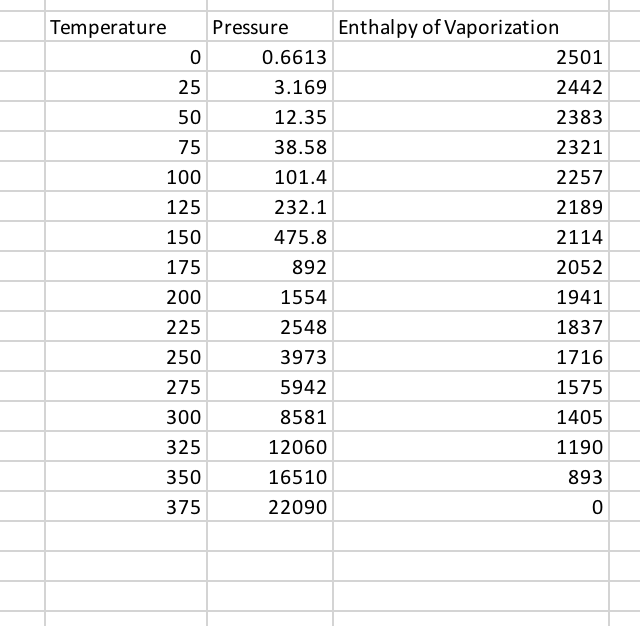
Molar enthalpy of vaporization of water from triple to critical points. | Download Scientific Diagram
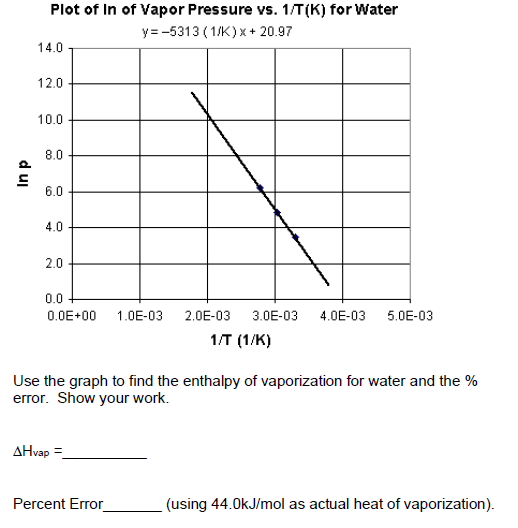
Calculate the molal elevation constant of water if molar enthalpy of vaporisation of water at 373 K is 40.585 kJ/mol.

Latent Heat of Vaporization – Delta Hvap – of Water calculated by corresponding states correlation in a one cell excel formula | Chem-Eng-Musings

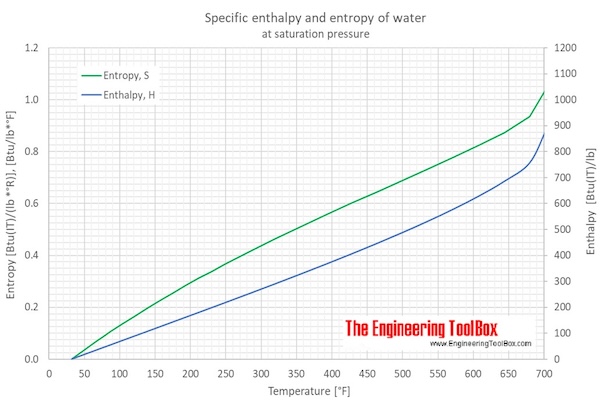
Enthalpy of vaporization of water: (—) Reference fundamental equation... | Download Scientific Diagram
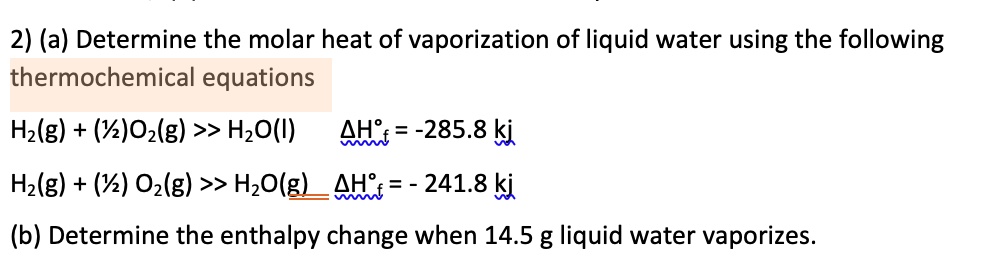
SOLVED: (a) Determine the molar heat of vaporization of liquid water using the following thermochemical equations H2(g) + 1/2O2(g) >> H2O(l) ΔH = -285.8 kJ H2(g) + 1/2O2(g) >> H2O(g) ΔH = -